Every year on 16th September, all the people around the world celebrate the significance of the Ozone layer as “The Ozone Day”. The importance of the ozone layer and the necessity to preserve it are brought to light by this day. It silently whispers in our ears and recalls our attention that towards the environment. It’s like a little nudge telling us that the planet has some big environmental problems. To keep it secure for next generations, we must work together. The importance of the ozone layer, what happens when it is damaged, and what steps are being taken to prevent further damage will all be covered in this blog article.
The Ozone Layer: Earth’s Protective Shield
Before going into the meaning of Ozone Day, find out more about the ozone layer and its significance. Three oxygen atoms make up the natural molecule ozone, which has the chemical formula O3. Ozone occurs in gaseous state and is found in layers of the Earth’s atmosphere. The majority, around 90%, of ozone is found between 15 and 30 kilometers above the Earth’s surface, and we call this part the stratospheric ozone. There’s also some ozone at ground level, but in smaller amounts, which we call tropospheric ozone. Unfortunately, when ozone is down here near the surface, it becomes a pollutant and is a significant component of the smog that often blankets cities.
The discovery of the ozone layer dates back to 1913 when French physicists Charles Fabry and Henri Buisson first identified it. Ozone (O3) molecules, which are relatively abundant in it and are essential for preserving life on our planet, are present in high concentrations. Ozone layer plays an important role as it prevents the harmful ultraviolet (UV) rays entering into the Earth’s atmosphere. Living things can be harmed by UV radiation since it can lead to a number of diseases such skin cancer, cataracts, and weakened immune systems. Additionally, UV radiation is capable of destroying marine ecosystems and the agricultural crops etc.
Ozone Depletion: What You Need to Know
Ozone layer depletion happens when the protective layer of ozone high up in the Earth’s atmosphere becomes thinner. This occurs because certain chemicals containing bromine or chlorine are released into the air from industries and human activities.
How Does Ozone Depletion Happen?
When chlorine and bromine atoms meet ozone in the sky, they break down ozone molecules. One single chlorine atom can destroy more than 100,000 ozone molecules before it leaves the sky.
Some chemicals release chlorine or bromine when they encounter strong UV light in the upper atmosphere. Ozone-depleting substances (ODS) are the name given to these compounds. Chemicals such as methyl chloroform, carbon tetrachloride, chlorofluorocarbons (CFCs), and hydrochlorofluorocarbons (HCFCs) falls under the category of ODS and are capable of producing chlorine. ODS that release bromine include substances like halons and methyl bromide. These dangerous substances are discharged onto the surface of the Earth, and it takes them two to five years to ascend to the upper atmosphere.
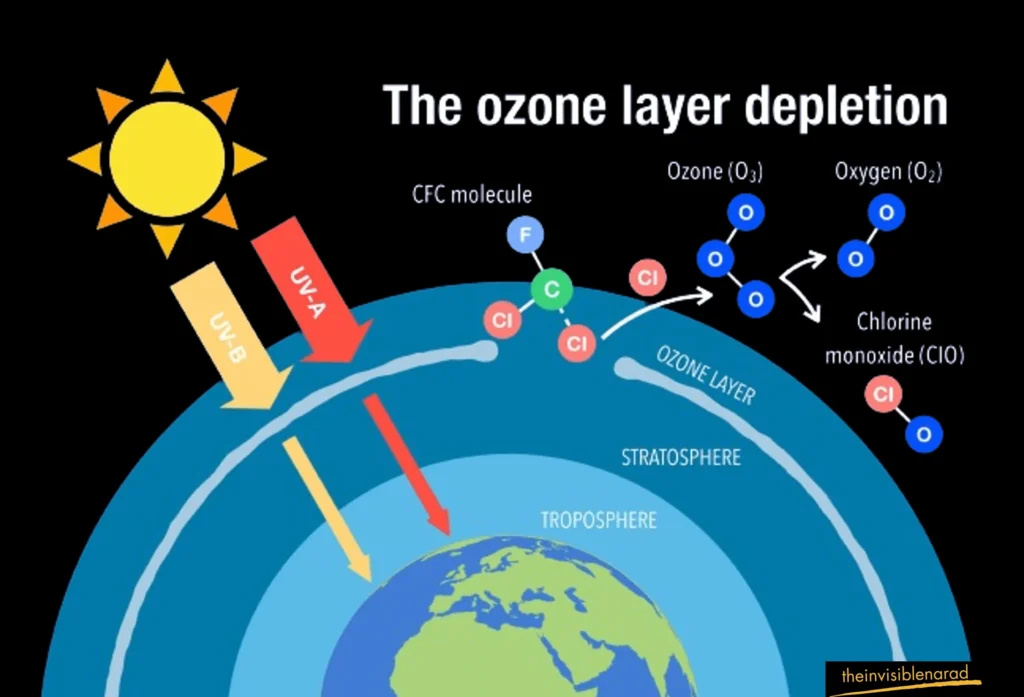
Natural events like big volcanic eruptions can also indirectly affect ozone levels. Apart from human activities volcanic eruptions are responsible for increasing the concentration of aerosols in the Earth’s atmosphere to a great extent. Aerosols are the suspended particles or droplets in the atmosphere. These Aerosols makes chlorine more effective at destroying ozone. However, this effect from volcanoes doesn’t last very long. The severe depletion of ozone creates the famous “ozone hole,” most visible in satellite images of the Antarctic ozone.
When Was Ozone Depletion Discovered?
In 1974, scientists Mario Molina and Frank Sherwood Rowland found a connection between CFCs and ozone breakdown in the upper atmosphere. In 1985, researchers Joe Farman, Brian G Gardiner, and Jon Shanklin reported unusually low ozone levels above Antarctica, which spurred global action. Their discoveries were supported by more investigation and study. In 1995, Molina, Rowland and Paul J. Crutzen were awarded Nobel Prize in Chemistry for their contributions.
The Consequences of Depletion of Ozone Layer
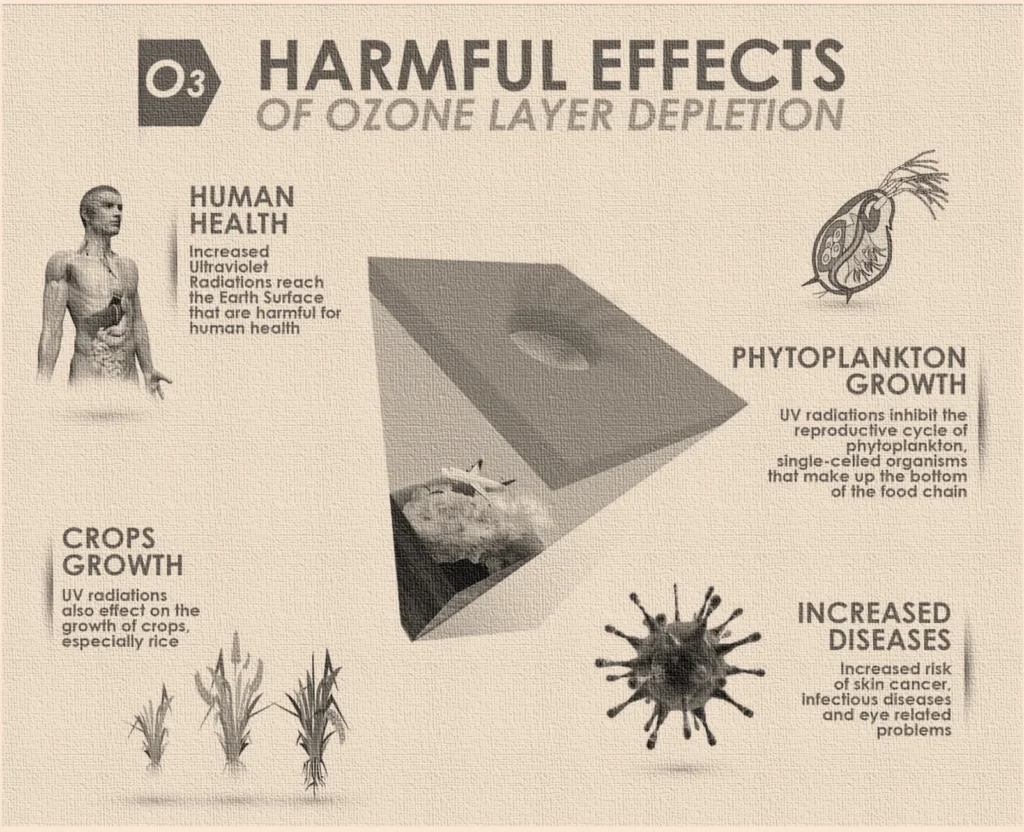
Human Health: More harmful UV radiation from the sun penetrates Earth’s atmosphere as the ozone layer thinning continues. In addition to causing skin cancer, including the deadly melanoma, this UV radiation can also cause cataracts.
On Plants
Increased UV radiation has an impact on plants as well. Although they can adapt to some UV light, excessive UV exposure might stunt their growth.
On Marine Ecosystem
Phytoplankton, one kind of marine life, may be impacted by UV radiation. This UV radiation tends to alter the behavior of these phytoplankton. Which in turn makes their survival difficult. There are many tiny and young marine species such as fishes and shrimps which are also impacted due to the imbalance created by loss of phytoplankton. This may throw off the ocean’s delicate balance of life.
On the Environment
More UV radiation can also mess with the way greenhouse gases like carbon dioxide that are in continuous circulation in the environment. Global Warming is one such impact of this UV rays Entering the Earth’s atmosphere. This further elevates the problems like rising sea level, melting glaciers, changing climate etc.
A Global Call to Action
Countries from all around the world made the decision to act to save the ozone layer in the late 1980s. They decide not to use all those substances that are harmful to the O3 layer. They took following measures:
The Vienna Convention
It took place in 1985, at Vienna Capital city of Austria. It was like the first step towards protecting the ozone layer. Although it failed to convince the countries to take some strong actions to protect the Ozone layer, but it paved the way for the Montreal Protocol.
The Montreal Protocol: A Global Agreement
In 1987, the world came together and made a special agreement called the Montreal Protocol. When the Montreal Protocol was made in 1987, it was a really special moment. Every country in the world agreed to it. This became a golden moment for the United Nations as it was the first time when all the countries agreed on a topic of concern as it was associated with our planet.
The main goal of the Montreal Protocol is to stop making and using substances that damage the ozone layer. It started with developed countries cutting back on a harmful substance called CFCs in 1993. They reduced their CFC use by 20% in 1994 and then by 50% in 1998 compared to what they used in 1986. They also stopped making a type of substance called halons as much as they did in 1986.
Later on, with course of time it was collectively decided to add some new rules to the Montreal Protocol. It was made sure that these rules were strong enough to put a check on harmful chemicals. It was also decided to take care off and help the developing countries who were ready to follow these rules. Few of these amendments are as follows:
The London Amendment (1990)
This one made it so that developed countries had to completely stop using CFCs, halons, carbon tetrachloride, and methyl chloroform by 2000. In developing countries, they had until 2010 to do this. They also put a cap on how much methyl bromide could be used, limiting it to the levels we used in 1991.
The Copenhagen Amendment (1992)
This amendment speed things up even more. It made developed countries get rid of CFCs, halons, carbon tetrachloride, and methyl chloroform by 1996. Plus, they started to phase out another harmful substance called HCFCs in 2004.
The Montreal Amendment (1997)
This one told developing countries to stop using HCFCs in 2005 and methyl bromide in 2015, just like developed countries.
The Beijing Amendment (1999)
It made stricter rules for the production and trade of HCFCs and added a new controlled substance called bromochloromethane.
The Kigali Amendment (2016)
The addition of hydrofluorocarbons (HFCs) to the list of prohibited chemicals made this a significant milestone. HFCs were substituted for O3-depleting ones by industries, however it turns out that they are equally hazardous for the climate. So, countries agreed to cut down on them too.
Healing the protective shield
Since countries started taking action through the Montreal Protocol, the use of chemicals that harm the ozone layer has gone down by about 98%. This has led to good news: the part of the sky where the harmful chemicals are decreasing, and the ozone layer is starting to recover.
In the early 2000s, experts predicted that the ozone layer would gradually improve with time. In the year 2000 the size of the O3 hole over Antarctica was at its largest. Its size was approximately 11.5 million square miles i.e 29.9 square kms. By 2021, it had gotten smaller, about 24.8 million square kilometers (9.6 million square miles).
According to a report published by United Nations in 2018, the ozone layer hole above Antarctica will gradually close. By the 2060s, levels will return to what it was in 1980.By the middle of the 2030s, the ozone layer over the Arctic, i.e., the North Pole, is anticipated to return to its 1980 levels. So, the O3 layer that protects us is doing better now!
Conclusion
The need of protecting the natural systems of the Earth is brought home by Ozone Day. Although we have made remarkable progress in healing the Ozone layer, but we have a long journey to cover. This small success should work like a motivator, and we should pledge to further reduce these harmful chemicals that harm our Ozone. The motive behind this day can only succeed if we spread this message, aware people around us about the importance of this layer. You can share this blog post among your friends, relatives and groups to aware them about the same.